Discovering a Genetic Mechanism that Affects Birth Defects, Some Cancers
Steven Vokes has studied the related Hedgehog signaling pathway for much of his career.
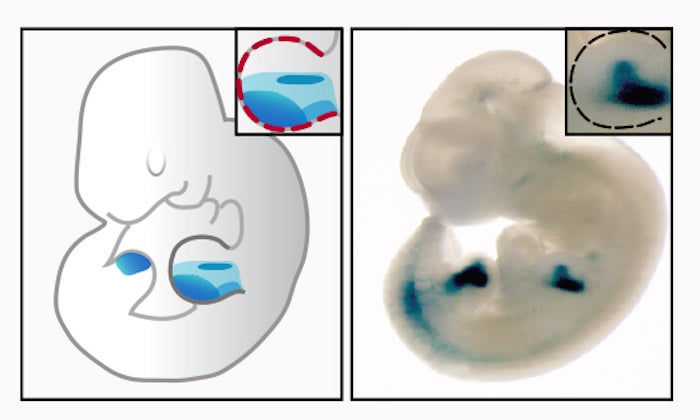
Images show Hedgehog responsive enhancer activity in tissue specific regions. Left: A mouse embryo with enhancer activity restricted to the developing limb buds. Right: A transgenic mouse embryo for one of the GLI-responsive enhancers. Images courtesy of VISTA enhancer database.
Scientists have understood for some time that proper embryonic development depends in large part on transcriptional repressors, proteins that prevent genes from being expressed at inappropriate times. Steven Vokes, associate professor of molecular biosciences at the University of Texas at Austin, and his team focus on a set of proteins called GLI (glioma-associated oncogene) and how they control gene expression in response to what is known as the Hedgehog pathway.
Vokes has studied the Hedgehog signaling pathway for much of his career. This pathway is responsible for transmitting the information to embryonic cells that is required for cell differentiation, allowing cells to become bones or muscles or brain cells. Mutations in the Hedgehog pathway or the genes it regulates cause a wide variety of birth defects in humans. The Hedgehog pathway also plays a role in regulating adult stem cells and in the maintenance and regeneration of adult tissues.
"This pathway is important from before the cradle to the grave, and it crosses so many different fields. It's one of the keys to the body's castle, so to speak," Vokes said. "The pathway itself is pretty well known, but the mechanisms at the DNA level are not."
Vokes studies the development of limbs in embryos and wanted to know how the Hedgehog pathway was involved in driving the growth of certain tissues. How does it tell cells to become limbs and how does it determine how many digits those limbs should have?
In a new paper out today in the journal eLife with researchers from Johns Hopkins University, the team details finding that the GLI repressors that keep the Hedgehog pathway turned off until the right time work by regulating chromatin (i.e., the stuff our chromosomes are made of like proteins, RNA, and DNA) on the level of enhancers, the important DNA sequences that act as hubs for activating gene expression. The Hedgehog pathway does this by regulating biochemical modifications on chromatin-associated proteins. These chromatin modifications inactivate enhancers which in turn prevent Hedgehog-regulated genes from being activated at the wrong time.
By gaining an understanding of this fundamental process, scientists may be able to unlock not only information about the genes casing specific birth defects but also new ways to target cancers in very specific ways. That's because the Hedgehog pathway also is implicated in the development of some cancers.
Medulloblastoma, for instance, is the most common type of brain cancer in children. Roughly 25 percent of these cancers are caused by a mutation in the Hedgehog-signaling gene.
"The hope for something like this is that you could take the genes that are causing this specific type of cancer and inactivate a specific enhancer that controls the expression of this gene solely in the brain. You could get much more targeted treatments," Vokes said.
Rachel Lex, Kristen Falkenstein and Joanna Henry of the University of Texas at Austin, as well as Zhicheng Ji, Weiqiang Zhou and Hongkai Ji of Johns Hopkins University contributed to the research. Lex, Zhicheng Ji and Falkenstein were co-first authors on the paper.
Funding for the research was provided by the National Institutes of Health and St. Baldrick's Foundation, a pediatric cancer research charity.